Microbial Barrier Test Astm . Validated as providing an effective barrier against microbial ingress. Comparison of this new microbial barrier test method vs. Porous sterile barrier integrity testing: False positives can occur when performing sterile integrity testing. The microbial barrier properties of a sus may be demonstrated using. Astm f1608, which is commonly called the log reduction value (lrv) test. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial.
from www.slideshare.net
False positives can occur when performing sterile integrity testing. Comparison of this new microbial barrier test method vs. The microbial barrier properties of a sus may be demonstrated using. Astm f1608, which is commonly called the log reduction value (lrv) test. Validated as providing an effective barrier against microbial ingress. Porous sterile barrier integrity testing: Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial.
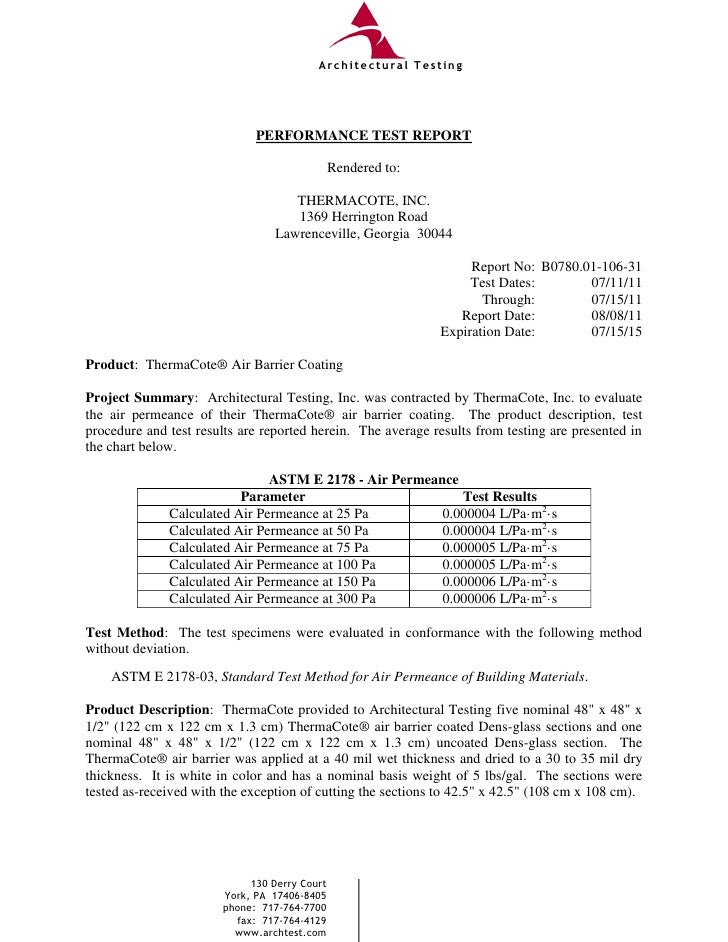
Therma cote air barrier test final report astm e 2178
Microbial Barrier Test Astm Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. The microbial barrier properties of a sus may be demonstrated using. Porous sterile barrier integrity testing: Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Comparison of this new microbial barrier test method vs. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. False positives can occur when performing sterile integrity testing. Validated as providing an effective barrier against microbial ingress.
From www.integratedpharmaservices.com
Microbial Barrier Testing of Novel Composite Dressings Microbial Barrier Test Astm The microbial barrier properties of a sus may be demonstrated using. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Validated as providing an effective barrier against microbial ingress. Test articles bearing calibrated defects over a range of dimensions, including. Microbial Barrier Test Astm.
From www.researchgate.net
Four components of the intestinal barrier. The microbiological barrier Microbial Barrier Test Astm The microbial barrier properties of a sus may be demonstrated using. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Astm f1608, which is commonly called the log reduction value (lrv). Microbial Barrier Test Astm.
From twitter.com
ASTM International on Twitter on pesticides, Microbial Barrier Test Astm Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Porous sterile barrier integrity testing: Comparison of this new microbial barrier test method vs. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Validated as providing an effective barrier against microbial. Microbial Barrier Test Astm.
From standards.iteh.ai
ASTM F263812e1 Standard Test Method for Using Aerosol Filtration for Microbial Barrier Test Astm Validated as providing an effective barrier against microbial ingress. The microbial barrier properties of a sus may be demonstrated using. Comparison of this new microbial barrier test method vs. False positives can occur when performing sterile integrity testing. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Astm f1608, which is commonly. Microbial Barrier Test Astm.
From www.science.org
Microbiota and maintenance of skin barrier function Science Microbial Barrier Test Astm Porous sterile barrier integrity testing: Astm f1608, which is commonly called the log reduction value (lrv) test. Validated as providing an effective barrier against microbial ingress. False positives can occur when performing sterile integrity testing. Comparison of this new microbial barrier test method vs. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size. Microbial Barrier Test Astm.
From gardening.gov.capital
How do microbial barriers control pests? Gardening.Gov.Capital Microbial Barrier Test Astm Validated as providing an effective barrier against microbial ingress. Porous sterile barrier integrity testing: The microbial barrier properties of a sus may be demonstrated using. Comparison of this new microbial barrier test method vs. False positives can occur when performing sterile integrity testing. Astm f1608, which is commonly called the log reduction value (lrv) test. Test articles bearing calibrated defects. Microbial Barrier Test Astm.
From nexusmedical.com
96Activation Microbial Barrier Performance Study Nexus Medical Microbial Barrier Test Astm Validated as providing an effective barrier against microbial ingress. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Comparison of this new microbial barrier test method vs. Porous sterile barrier integrity testing: Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. Microbial Barrier Test Astm.
From standards.iteh.ai
ASTM F263822 Standard Test Method for Using Aerosol Filtration for Microbial Barrier Test Astm Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Comparison of this new microbial barrier test method vs. The microbial barrier properties of a sus may be demonstrated using. False positives can occur when performing sterile integrity testing. Validated as. Microbial Barrier Test Astm.
From www.youtube.com
ASTM D4541 Air Barrier Adhesion Test Pulloff Strength YouTube Microbial Barrier Test Astm Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Comparison of this new microbial barrier test method vs. False positives can occur when performing sterile integrity testing. The microbial barrier properties of a sus may be. Microbial Barrier Test Astm.
From www.researchgate.net
Schematic of microbial barrier permeability testing. Download Microbial Barrier Test Astm Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Comparison of this new microbial barrier test method vs. Validated. Microbial Barrier Test Astm.
From www.plasticsurgery.theclinics.com
Microbial Barriers Clinics in Plastic Surgery Microbial Barrier Test Astm Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. The microbial barrier properties of a sus may be demonstrated using. Porous sterile barrier integrity testing: Comparison of this new microbial barrier test method vs. Validated as providing an effective barrier against microbial ingress. Test articles bearing calibrated defects over a range of. Microbial Barrier Test Astm.
From airbarriertesting.blogspot.com
Air Barrier Testing (ASTM E1827 and ASTM E779) Microbial Barrier Test Astm The microbial barrier properties of a sus may be demonstrated using. Validated as providing an effective barrier against microbial ingress. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Porous sterile barrier integrity testing: Comparison of this new microbial barrier. Microbial Barrier Test Astm.
From www.slideshare.net
Therma cote air barrier test final report astm e 2178 Microbial Barrier Test Astm Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Validated as providing an effective barrier against microbial ingress. Porous sterile barrier integrity testing: The microbial barrier properties of a sus may be demonstrated using. Two test. Microbial Barrier Test Astm.
From standards.iteh.ai
ASTM F263818 Standard Test Method for Using Aerosol Filtration for Microbial Barrier Test Astm The microbial barrier properties of a sus may be demonstrated using. Validated as providing an effective barrier against microbial ingress. Astm f1608, which is commonly called the log reduction value (lrv) test. Porous sterile barrier integrity testing: False positives can occur when performing sterile integrity testing. Test articles bearing calibrated defects over a range of dimensions, including up to a. Microbial Barrier Test Astm.
From standards.iteh.ai
ASTM F263807 Standard Test Method for Using Aerosol Filtration for Microbial Barrier Test Astm False positives can occur when performing sterile integrity testing. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Comparison of this new microbial barrier test method vs. Validated as providing an effective barrier against microbial ingress. The microbial barrier properties of a sus may be demonstrated using. Astm. Microbial Barrier Test Astm.
From www.researchgate.net
Schematic representation of testing microbial barrier permeability Microbial Barrier Test Astm Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. Porous sterile barrier integrity testing: Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. Astm f1608, which is commonly called the log reduction value (lrv) test. Validated as providing an effective. Microbial Barrier Test Astm.
From www.dupont.co.jp
Microbial Barrier DuPont™ Tyvek® for Medical Packaging DuPont USA Microbial Barrier Test Astm Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial. False positives can occur when performing sterile integrity testing. Comparison of this new microbial barrier test method vs. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. The microbial barrier properties. Microbial Barrier Test Astm.
From standards.iteh.ai
ASTM F263807 Standard Test Method for Using Aerosol Filtration for Microbial Barrier Test Astm Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial. False positives can occur when performing sterile integrity testing. Comparison of this new microbial barrier test method vs. Porous sterile barrier integrity testing: Astm f1608, which is commonly called the log reduction value (lrv) test. Test articles bearing calibrated defects over a range. Microbial Barrier Test Astm.